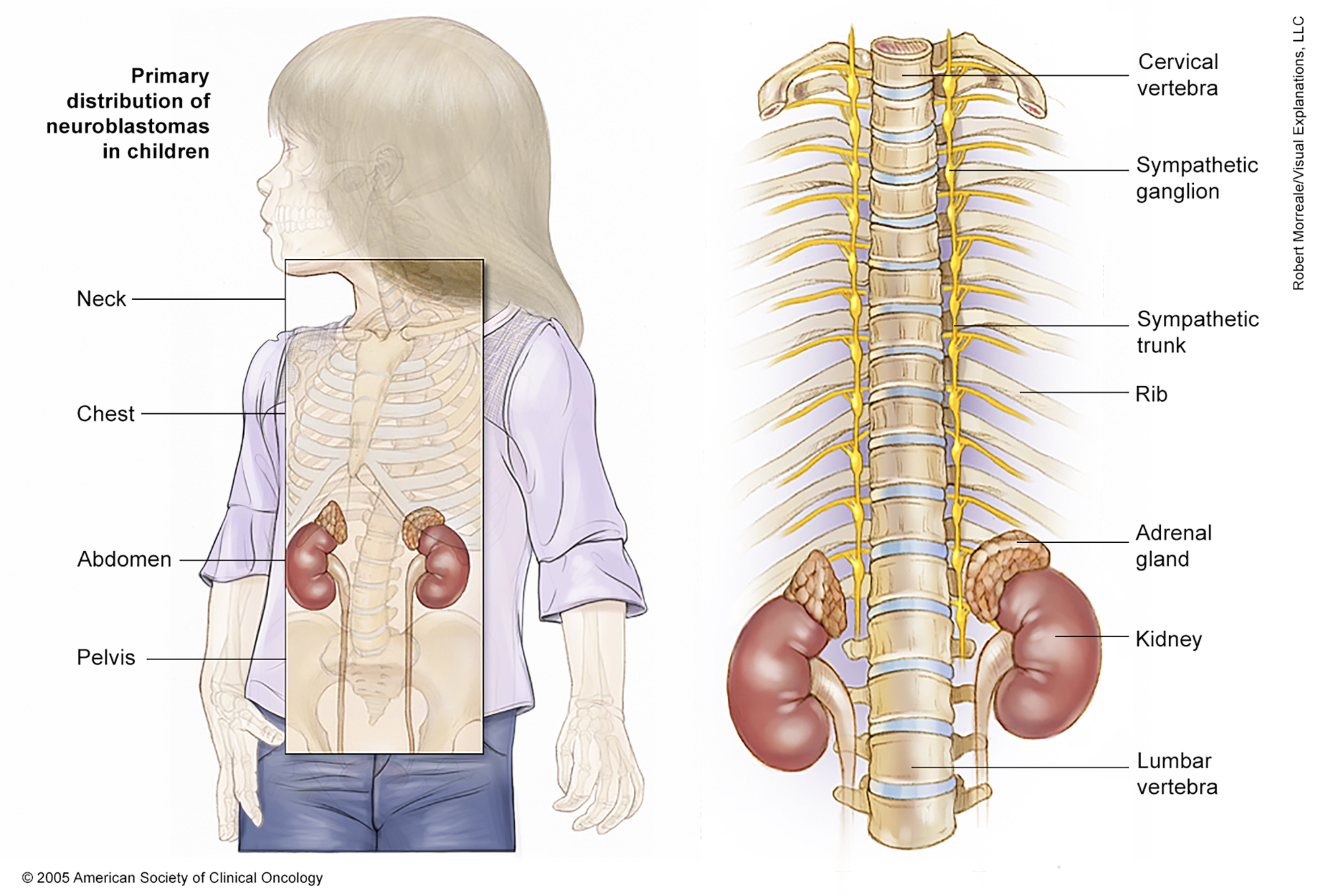
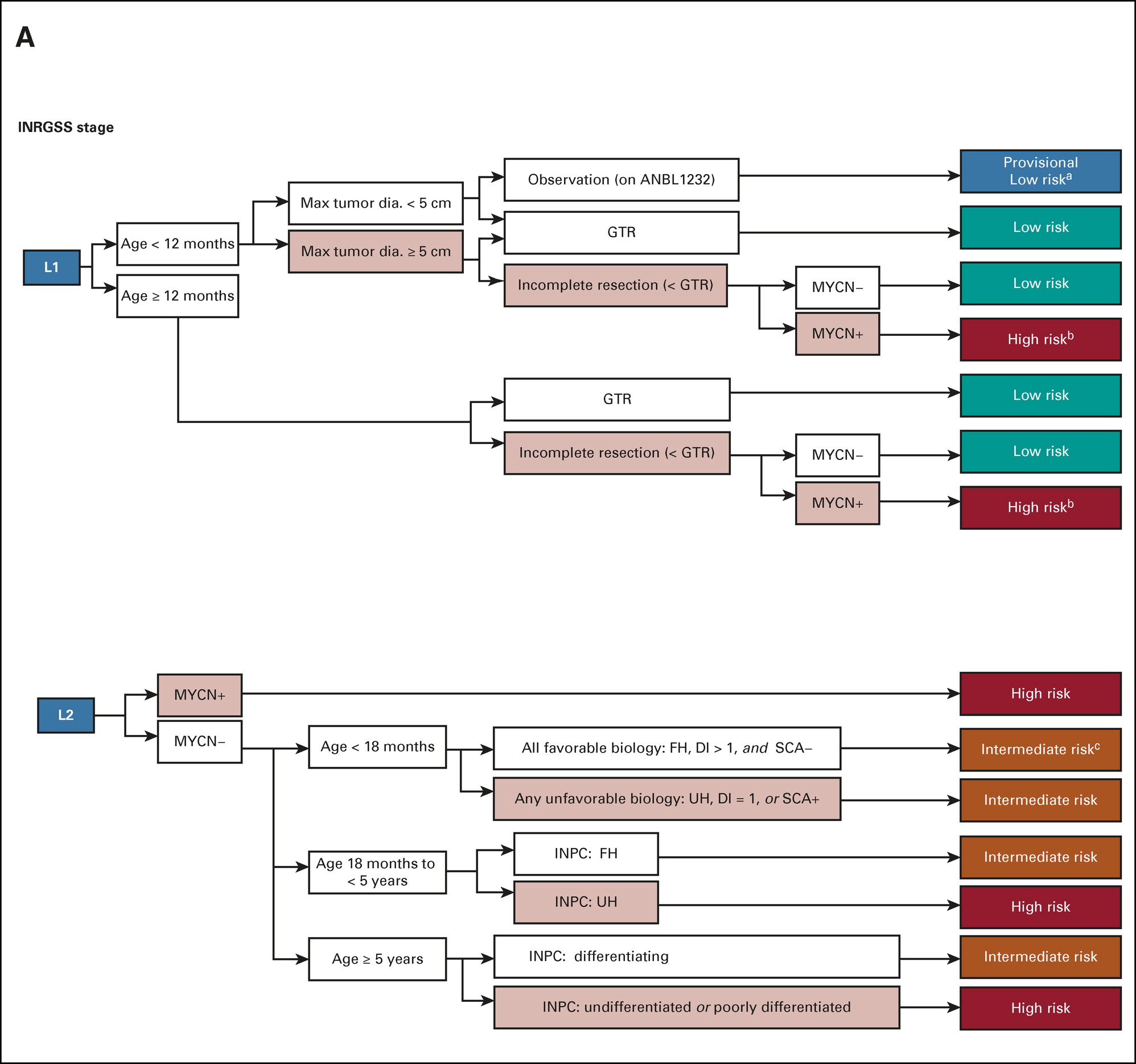
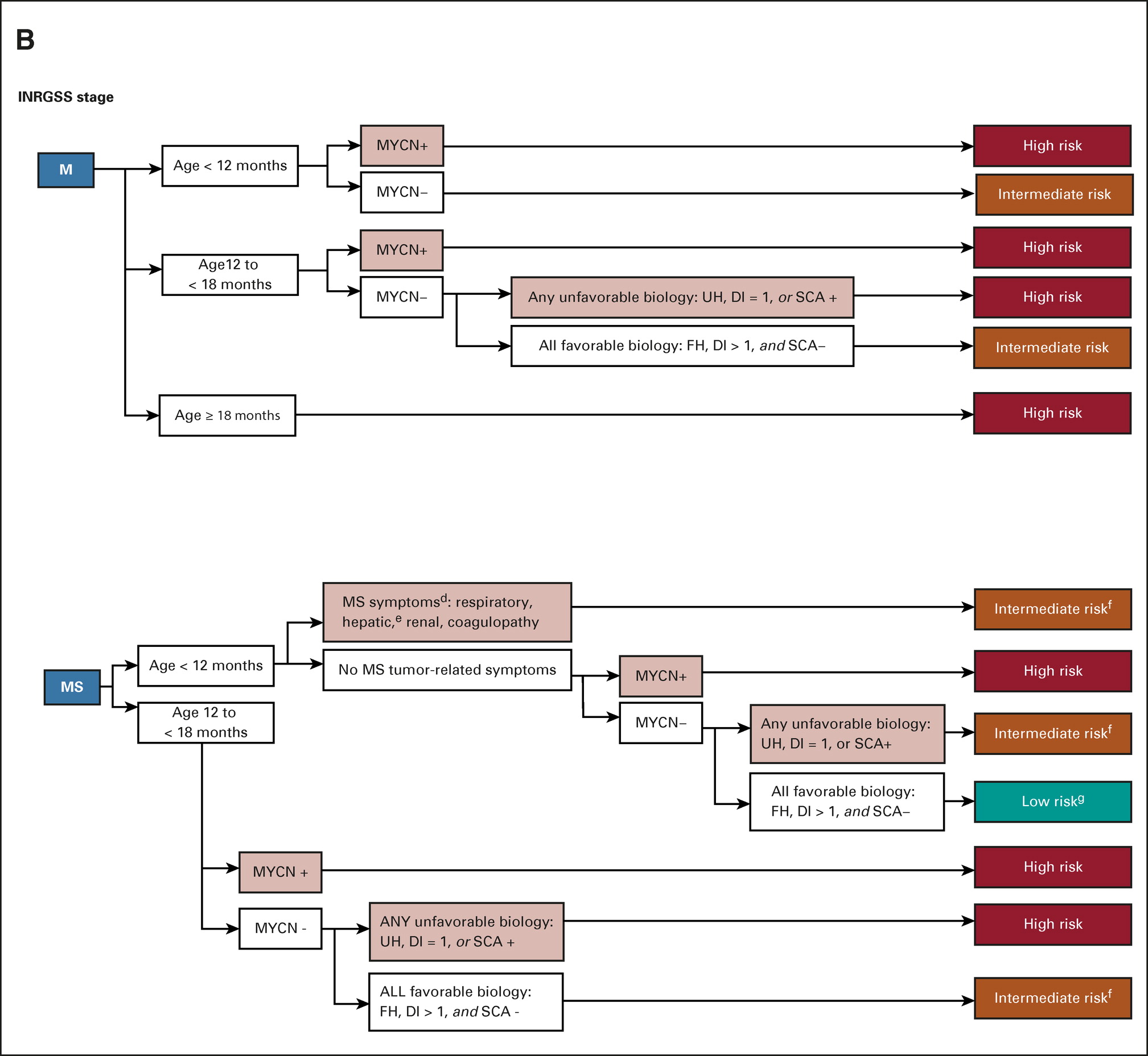
ON THIS PAGE: You will learn about the different treatments doctors use for children with neuroblastoma. Use the menu to see other pages.
In general, cancer in children is uncommon. This means it can be hard for doctors to plan treatments unless they know what has been most effective in other children. A clinical trial is a research study that tests a new approach to treatment. The “standard of care” is the best treatments known based on previous clinical trial results. Clinical trials may test such approaches as a new drug, a new combination of existing treatments, or new doses of current therapies. The health and safety of all children participating in clinical trials are closely monitored.
To take advantage of these newer treatments, children with cancer should be treated at a specialized cancer center. Doctors at these centers have extensive experience in treating children with cancer and have access to the latest research. A doctor who specializes in treating children with cancer is called a pediatric oncologist. If a pediatric cancer center is not nearby, general cancer centers sometimes have pediatric specialists who are able to be part of your child’s care.
How neuroblastoma is treated
In many cases, a team of doctors works with a child and the family to provide care. This is called a multidisciplinary team. Pediatric cancer centers often have extra support services for children and their families, such as child life specialists, dietitians, physical therapists, occupational therapists, social workers, and counselors. Special activities and programs to help your child and family cope may also be available. Learn more about the clinicians who provide cancer care.
Treatment options and recommendations for neuroblastoma depend on several factors, including:
-
The patient's risk classification
-
The size and location of the tumor
-
Whether the cancer has spread
-
Possible side effects of treatment
-
Family preferences
-
The child’s overall health
In particular, the treatment plan is tailored based on the assigned risk group.
Take time to learn about all of your child’s treatment options and be sure to ask questions about things that are unclear. Talk with your child’s doctor about the goals of each treatment and what your child can expect while receiving the treatment. These types of conversations are called “shared decision-making.” Shared decision-making is when you and your doctors work together to choose treatments that fit the goals of your child’s care. Shared decision-making is important for childhood neuroblastoma because there are different treatment options. Learn more about making treatment decisions.
Descriptions of the most common treatment options for neuroblastoma are described below. Your child’s care plan also includes treatment for symptoms and side effects, an important part of cancer care. Learn more about preparing your child for treatment.
READ MORE BELOW:
Observation/wait and watch
A small group of infants with localized neuroblastoma do not require any active treatment, including surgery. Instead, the health of these patients is monitored closely by their doctors, an approach called "observation" or "wait and watch."
A previous Children’s Oncology Group study has demonstrated that it is safe to closely observe some infants younger than 6 months of age with a small tumor using imaging scans, physical exams, and laboratory tests. Over time, the majority of these neuroblastoma tumors regressed without further treatment. If there was evidence of tumor growth, the infants were then treated with surgery with or without chemotherapy, and all the patients enrolled on this clinical trial survived. In a Children's Oncology Group study that was recently closed to accrual, a wait and watch approach was investigated for a broader population of patients, including those with International Neuroblastoma Risk Group Staging System (INRGSS; see Stages and Risk Groups) stage L1 tumors who are younger than 12 months of age at diagnosis with a tumor smaller than 5 centimeters located in adrenal or non-adrenal areas of the body.
Return to top
Surgery
Surgery is the removal of the tumor and some surrounding healthy tissue during an operation. A surgical oncologist is a doctor who specializes in treating cancer using surgery. If the cancer has not spread, surgery can sometimes be used to remove the entire tumor. However, most neuroblastoma is not found until after the cancer has spread. In that situation, the doctor can remove as much of the tumor as possible during surgery.
Even if a tumor cannot be removed because of its location, a surgical biopsy (see Diagnosis) may still be done to determine the type of tumor.
If the tumor cannot be completely removed with surgery, the child may receive radiation therapy and chemotherapy (see below) after surgery to destroy the remaining cancer cells. In addition, the doctor may take a biopsy of the liver to find out if the disease has spread to the liver.
Talk with your child’s surgeon about the possible side effects of the surgery and how they will be relieved or managed. Learn more about the basics of cancer surgery.
Return to top
Chemotherapy
Chemotherapy is the use of drugs to destroy cancer cells, usually by keeping the cancer cells from growing, dividing, and making more cells. Chemotherapy is given by a pediatric oncologist or a medical oncologist, a doctor who specializes in treating cancer with medication.
Systemic chemotherapy gets into the bloodstream to reach cancer cells throughout the body. Common ways to give chemotherapy include an intravenous (IV) tube placed into a vein or muscle using a needle or in a pill or capsule that is swallowed by mouth. If your child is prescribed oral medications to take at home, be sure to ask your health care about how to safely store and handle them. A chemotherapy regimen (schedule) usually consists of a specific number of cycles given over a set period of time.
Most children with neuroblastoma will need to have chemotherapy. Chemotherapy may be used as the primary treatment for neuroblastoma. Or, it may be given before surgery to shrink the tumor or after surgery to destroy any remaining cancer cells.
Children with intermediate-risk neuroblastoma often receive the following drugs:
Patients with high-risk neuroblastoma are treated with 3 phases of therapy. "Induction chemotherapy" is administered after the diagnosis is confirmed to destroy as many of the cancer cells as possible. Stem cell harvest and surgery are also conducted during the induction phase of therapy. "Consolidation therapy" begins after induction chemotherapy has been completed. During consolidation therapy, patients are treated with high-dose chemotherapy followed by stem cell transplant and radiation therapy. Standard-of-care consolidation therapy in North America includes 2 cycles of high-dose therapy and stem cell transplant. Following consolidation, patients are treated with immunotherapy and isotretinoin.
Children with high-risk neuroblastoma often receive the following drugs:
-
Busulfan (Busulfex, Myleran)
-
Carboplatin (Paraplatin)
-
Cisplatin (Platinol)
-
Cyclophosphamide (Neosar)
-
Cytokines (GM-CSF and IL2)
-
Dinutuximab (Unituxin)
-
Doxorubicin (Adriamycin, Doxil)
-
Etoposide (VePesid, Toposar)
-
Ifosfamide (Ifex)
-
Isotretinoin
-
Melphalan (Alkeran)
-
Thiotepa
-
Topotecan (Hycamtin)
-
Vincristine (Vincasar)
The side effects of chemotherapy depend on the individual and the dose used, but they can include fatigue, risk of infection, nausea and vomiting, hair loss, loss of appetite, and diarrhea. These side effects usually go away once treatment is finished. The severity of the side effects depends on the type and amount of the drug being given and the length of time the child receives the drug.
Learn more about the basics of chemotherapy and preparing for treatment. The medications used to treat cancer are continually being evaluated. Talking with your child’s doctor is often the best way to learn about the medications prescribed for you, their purpose, and their potential side effects or interactions with other medications. Learn more about your prescriptions by using searchable drug databases.
Return to top
Radiation therapy
Radiation therapy is the use of high-energy x-rays or other particles to destroy cancer cells. A doctor who specializes in giving radiation therapy to treat cancer is called a radiation oncologist. The most common type of radiation treatment is called external-beam radiation therapy, which is radiation given from a machine outside the body. Based on the location of the tumor, proton therapy may also be recommended. Proton therapy is a type of external-beam radiation therapy that uses protons rather than x-rays. At high energy, protons can destroy cancer cells.
A radiation therapy regimen, or schedule, usually consists of a specific number of treatments given over a set period of time. Side effects from radiation therapy may include fatigue, mild skin reactions, upset stomach, and loose bowel movements. Most side effects go away soon after treatment is finished. Talk with the radiation oncologist about what side effects your child can expect and how they will be managed.
Because radiation therapy can sometimes cause problems with the normal growth and development of a child’s brain, ovaries, or testicles, the doctor may choose to treat the cancer in another way.
Learn more about the basics of radiation therapy.
Return to top
Stem cell transplantation
An autologous (AUTO) stem cell transplant is also called a hematopoietic stem cell transplant. This is a medical procedure in which highly specialized cells, called hematopoietic stem cells, are collected from the patient's bloodstream. The stem cells are then frozen until they are needed. Then the patient is given high doses of chemotherapy to treat the neuroblastoma. After that, the collected hematopoietic stem cells, or blood-forming cells, are infused back into the patient.
Before recommending a transplant, doctors will talk with the patient and family about the risks of this treatment. They will also consider several other factors, such as the type of cancer, results of any previous treatment, and the patient’s age and general health.
The goal of a transplant is to destroy cancer cells in the bone marrow, blood, and other parts of the body and allow replacement blood stem cells to create healthy bone marrow. During the process, the patient is treated with high doses of chemotherapy to destroy as many cancer cells as possible. However, this also destroys the patient’s bone marrow tissue and suppresses the immune system. After the high-dose treatment is given, blood stem cells are infused into the patient’s vein to replace the bone marrow and restore normal blood counts.
For neuroblastoma, different combinations of high-dose chemotherapy have been used before the stem cells are infused. A European clinical trial demonstrated that patients who received busulfan (Myleran, Busilvex) and melphalan (Alkeran, Evomela) had improved event-free survival and fewer side effects than children who were treated with carboplatin (Paraplatin), etoposide (available as a generic drug), and melphalan. Event-free survival is the measure of time after treatment that a group of people in a clinical trial has not had their cancer come back or get worse.
At the same time the European study was ongoing, the Children’s Oncology Group conducted a study comparing a single cycle of high-dose chemotherapy (carboplatin, etoposide, and melphalan) with stem cell transplant, giving tandem cycles of high-dose chemotherapy with stem cell transplant after each cycle of chemotherapy. The first cycle of consolidation consisted of cyclophosphamide (available as a generic drug) and thiotepa, and the second consisted of carboplatin, etoposide, and melphalan. The results of this Children’s Oncology Group clinical trial demonstrated improved event-free survival for those patients who received 2 cycles of high-dose chemotherapy and stem cell transplant. Based on the results of this study, tandem cycles of high-dose chemotherapy with a stem cell transplant are now considered part of the standard treatment for children with high-risk neuroblastoma.
In an AUTO transplant, there is little risk of tissue rejection because the replacement stem cells are the patient’s own cells, rather than from a donor. However, there is a risk that some of the cells that are put back into the patient could still be cancerous.
Side effects depend on the type of transplant, your child’s general health, and other factors. Learn more about the basics of stem cell transplant.
Return to top
Immunotherapy
Immunotherapy uses the body's natural defenses to fight cancer by improving your immune system’s ability to attack cancer cells.
GD2 is a compound on the surface of cells, called a disialoganglioside, that is found in large amounts in most neuroblastomas. A variety of monoclonal antibodies directed against GD2 have been used to treat neuroblastoma. A monoclonal antibody is a substance made in a laboratory that acts like the antibodies the body’s immune system naturally makes to fight diseases such as cancer.
The Children's Oncology Group conducted a clinical trial in the early 2000s testing a chimeric (part human, part mouse) anti-GD2 monoclonal antibody therapy (dinutuximab) combined with cytokines (namely, GM-CSF and IL-2), which are proteins that stimulate the immune system, and retinoid therapy (see below) versus retinoic acid (RA) alone for patients with high-risk neuroblastoma when:
-
The neuroblastoma responded to induction chemotherapy, and
-
The patient underwent a stem cell transplant (see above) without the neuroblastoma growing or spreading.
Patients with biopsy-proven residual (leftover) neuroblastoma following a stem cell transplant were non-randomly assigned to receive immunotherapy as part of the study.
Among the patients enrolled on this clinical trial who were randomized, those who received immunotherapy and RA had higher rates of survival and fewer recurrences than those who received the RA alone. Immunotherapy and RA is now a part of the standard treatment for patients with high-risk neuroblastoma. Based on the results of this study, the U.S. Food and Drug Administration (FDA) approved dinutuximab (Unituxin) in 2015 as part of first-line therapy for children with high-risk neuroblastoma. Long-term follow-up of the randomized patients demonstrated that immunotherapy with dinutuximab improved the 5-year survival. Outcomes of more than 1,000 high-risk patients treated with dinutuximab on this clinical trial after randomization was stopped confirmed the survival and toxicity outcomes.
In the multi-center SIOPEN clinical trial, the Chimeric14.18 anti-GD2 monoclonal antibody manufactured in animal cells (dinutuximab beta) with or without the IL2 cytokine was evaluated in a randomized clinical trial. The results of this study showed there was no evidence that subcutaneous IL-2 immunotherapy with dinutuximab beta improved event-free survival in patients with high-risk neuroblastoma that responded to standard induction and consolidation treatment. Based on these results, IL-2 is no longer given with dinutuximab in Children's Oncology Group studies, and standard post-consolidation immunotherapy consists of dinutuximab plus GM-CSF and RA.
Humanized versions of the anti-GD2 monoclonal antibody, including naxitamab, a humanized version of the mouse monoclonal anti-GD2 antibody 3F8, and Hu14.18K322A are also being tested in clinical trials. A phase 2 single-institution study of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma showed promising rates of a partial response or better, meaning the tumor had shrunk. In this study, HU14.18K322A was also combined with consolidation treatment and administered following consolidation, and encouraging 3-year event-free survival was observed. A recent Children's Oncology Group pilot study has shown it is feasible to combine dinutuximab with induction chemotherapy in the cooperative group setting. A randomized phase 3 Children's Oncology Group study testing the efficacy of dinutuximab combined with induction chemotherapy is being developed and scheduled to open to patient enrollment in 2024.
Different types of immunotherapy can cause different side effects. Common side effects include skin reactions, flu-like symptoms, diarrhea, and weight changes. Anti-GD2 antibodies commonly cause severe pain, and narcotics are used to relieve this pain. Other common side effects include low blood pressure, called hypotension, and allergic reactions. Talk with your child’s doctor about what to expect with possible side effects for the immunotherapy recommended for your child. Learn more about the basics of immunotherapy.
Return to top
Retinoid therapy
Retinoids are substances that are similar to vitamin A. They are thought to help some cells mature into normal cells. In a previous Children’s Oncology Group clinical trial, high-risk patients with neuroblastoma who had no evidence of active disease following completion of consolidation therapy received 13-cis-retinoic acid (RA) (isotretinoin) versus no further treatment. The study showed that the children who received RA had improved event-free survival.
Return to top
Targeted therapy (updated 12/2023)
Targeted therapy is a treatment that targets the cancer’s specific genes, proteins, or the tissue environment that contributes to cancer growth and survival. This type of treatment blocks the growth and spread of cancer cells while limiting damage to healthy cells.
Not all cancers have the same targets. To find the most effective treatment, your child's doctor may run tests to identify the genes, proteins, and other factors involved in your child's cancer. This helps doctors better match each patient with the most effective treatment whenever possible. In addition, research studies continue to find out more about specific molecular targets and new treatments directed at them. Learn more about the basics of targeted treatments.
Tyrosine kinase inhibitors
Anaplastic lymphoma kinase (ALK) is a normal part of the cell growth process. When present, a change or mutation in the ALK gene helps cancer cells grow. ALK inhibitors help stop this process. ALK mutations have been identified in the germline (inherited) genes of a person and/or the tumor's genes in a subset of patients with neuroblastoma. Drugs that target ALK are called tyrosine kinase inhibitors (TKIs). TKIs are a type of targeted therapy. A number of ALK small-molecule TKIs have been developed and tested in early-phase clinical trials in children when the neuroblastoma continues to grow despite treatment, called refractory disease, or when the neuroblastoma returns following treatment, called recurrent disease.
One study, which is a phase 3 clinical trial from the Children’s Oncology Group for those with high-risk disease, is testing whether adding the ALK inhibitor lorlatinib (Lorbrena) to standard of care treatment helps patients with newly diagnosed neuroblastoma with ALK mutations.
Talk with your doctor about all clinical trials that may be available to your child as part of your treatment decision-making. Also, be sure to talk with your doctor about possible side effects from the specific targeted therapy recommended for your child and ways to manage side effects.
Ornithine decarboxylase 1 (ODC1) inhibitor
This is another type of targeted therapy. Ornithine decarboxylase 1 (ODC1) is an important enzyme that contributes to cell survival. In laboratory studies, an ODC inhibitor called eflornithine (Iwilfin, DFMO) slowed the growth of neuroblastoma tumors in mice. For high-risk neuroblastoma, a single-arm phase II study investigated adding eflornithine to treatment after immunotherapy was completed. Analysis of the study's results showed that patients with high-risk neuroblastoma who received eflornithine after completing immunotherapy had better rates of slowing or stopping the disease and lived longer than those who did not receive this additional treatment.
In 2023, the FDA approved eflornithine as treatment to reduce the risk of recurrence (relapse) in adults and children with high-risk neuroblastoma. Eligible patients must have had at least a partial response to previous treatments with multiple medications, including anti-GD2 immunotherapy. This represents the first FDA approval of a medication intended to reduce the risk of recurrence in children with high-risk neuroblastoma.
Return to top
Targeted delivery of radionuclides
A radionuclide called 131I-MIBG has been used to treat children when the neuroblastoma recurs, and tumor responses have been seen in about 30% of these children. Based on these results, a clinical trial was started by the Children’s Oncology Group testing the effectiveness of using 131I-MIBG plus an AUTO stem cell transplant followed by busulfan and melphalan as consolidation therapy for newly diagnosed patients with high-risk disease. The Children’s Oncology Group study is currently conducting a randomized phase 3 clinical trial for this group of patients to test the effectiveness of integrating 131I-MIBG in the induction phase of treatment.
Return to top
Physical, emotional, social, and financial effects of cancer
Neuroblastoma and its treatment cause physical symptoms and side effects, as well as emotional, social, and financial effects. Managing all of these effects is called palliative and supportive care. It is an important part of your child’s care that is included along with treatments intended to slow, stop, or eliminate the cancer.
Palliative and supportive care focuses on improving how your child feels during treatment by managing symptoms and supporting patients and their families with other, non-medical needs. Any person, regardless of age or type and stage of cancer, may receive this type of care. And it often works best when it is started right after a cancer diagnosis. People who receive palliative and supportive care along with treatment for the cancer often have less severe symptoms, better quality of life, and report that they are more satisfied with treatment.
Palliative treatments vary widely and often include medication, nutritional changes, relaxation techniques, emotional and spiritual support, and other therapies. Your child may also receive palliative treatments, such as cancer medications, surgery, or radiation therapy, to improve symptoms.
Before treatment begins, talk with your child’s doctor about the goals of each treatment in the recommended treatment plan. You should also talk about the possible side effects of the specific treatment plan and palliative and supportive care options. Many patients also benefit from talking with a social worker and participating in support groups. Ask your doctor about these resources, too.
Cancer care is often expensive, and navigating health insurance can be difficult. Ask your child's doctor or another member of the health care team about talking with a financial navigator or counselor who may be able to help with your financial concerns.
During treatment, your child’s health care team may ask you to answer questions about your child’s symptoms and side effects and to describe each problem. Be sure to tell the health care team if your child is experiencing a problem. This helps the health care team treat any symptoms and side effects as quickly as possible. It can also help prevent more serious problems in the future.
Learn more about the importance of tracking side effects in another part of this guide. Learn more about palliative and supportive care in a separate section of this website.
Return to top
Remission and the chance of recurrence
A remission is when a tumor cannot be detected in the body and there are no symptoms. This may also be called having “no evidence of disease” or NED.
A remission may be temporary or permanent. This uncertainty causes many people to worry that their cancer will come back. While many remissions are permanent, it is important to talk with your doctor about the possibility of the tumor returning. Understanding your child’s risk of recurrence and the treatment options may help you and your family feel more prepared if the cancer does return. Learn more about coping with the fear of recurrence.
If neuroblastoma comes back after the original treatment, it is called a recurrent or relapsed cancer. It may come back in the same place (called a local recurrence), nearby (regional recurrence), or in another place (distant recurrence). If there is a recurrence, the cancer may need to be staged again using the system described in Stages and Risk Groups.
If a recurrence happens, a new cycle of testing will begin to learn as much as possible about it. After this testing is done, you and your child’s doctor will talk about the treatment options. Often the treatment plan will include the treatments described above, such as surgery, cancer medications, and radiation therapy, but they may be used in a different combination or given at a different pace. Your child’s doctor may suggest clinical trials that are studying new ways to treat recurrent neuroblastoma.
Treatment of refractory or recurrent neuroblastoma
Refractory cancer is cancer that continues to grow despite treatment. As explained above, recurrent cancer is cancer that comes back after treatment.
The treatment of refractory and recurrent neuroblastoma depends on the location of the previous treatment, tumor biology, the risk group at diagnosis, and whether there are certain gene mutations. There are treatments that work well for patients with low-risk and intermediate-risk disease who have a recurrence where the original tumor started. Recurrent high-risk neuroblastoma remains difficult to treat successfully. Neuroblastoma comes back in approximately 40% to 50% of children with high-risk disease.
In recent years, more neuroblastoma treatments and new combinations of treatments have been developed or are being researched. This includes treatment options available through clinical trials. Many of the approaches below are explained above, for additional details.
-
Anti-GD2 antibody immunotherapy. A number of anti-GD2 antibodies have been developed and are being tested in studies for patients with recurrent or refractory neuroblastoma. In a Children’s Oncology Group phase 2 clinical trial testing the combination of chemotherapy (irinotecan and temozolomide) plus dinutuximab, tumor responses were seen in about 40% of children with recurrent or refractory neuroblastoma. The Children's Oncology Group is currently conducting a study for patients with recurrent or refractory disease testing the addition of difluoromethylornithine (DFMO) to the chemoimmunotherapy regimen. A European (SIOPEN) BEACON clinical trial is testing the combination of chemotherapy and dinutuximab-beta. Single-institution studies have indicated that, for a subset of patients, some refractory and relapsed high-risk neuroblastoma will respond to the humanized anti-GD2 antibody naxitamab. Promising responses have also been observed with naxitamab combined with temozolomide and irinotecan and GM-CSF in a single-institution phase 2 study. Other anti-GD2 antibodies that have been developed include Hu14.18K332A and dinutuximab-beta. The efficacy of the bispecific antibody Hu3F8-BsAb and the cytokine-antibody fusion molecule Hu14.18-IL2 are also being evaluated. Dinutuxmiab is also being evaluated in combination with natural killer (NK) cells and the immune-modulating agent lenolinomide in a New Approaches to Neuroblastoma Therapy (NANT) phase 1 clinical trial. Dinutuximab combined with 131I-MIBG and vorinostat is also being tested in an ongoing NANT phase 1 clinical trial.
-
Targeted delivery of radionuclides. Radionuclides have been attached to MIBG, as well as somatostatin analogs, which are substances similar to a specific hormone produced by cells. 131I-MIBG therapy has been shown to be active in patients with recurrent or refractory neuroblastoma with response rates of approximately 30%. A recently completed NANT clinical trial testing the efficacy of 131I-MIBG alone or MIBG combined with radiation sensitizers irinotecan or vorinostat (Zolinza) demonstrated higher rates of response with 131I-MIBG combined with vorinostat. Radiosensitizers are drugs that make tumor cells more sensitive to radiation therapy, which makes radiation therapy more effective. Additional studies are now testing the combination of 131I-MIBG and dinutuximab and vorinostat in patients with recurrent or refractory neuroblastoma.
-
Immunotherapies. Early-phase studies testing immune checkpoint inhibitors alone or in combination with MIBG and dinutuximab are ongoing. Additional phase 1 studies are testing T cells engineered to express chimeric antigen receptors (CARs) targeting GD2, LICAM, B7-H3, or other targets. This is called CAR T-cell therapy. The efficacy of anti-GD2 vaccines is also being evaluated in patients who achieve a complete response with other treatments in a single-institution study.
-
Therapies that target the ALK oncogene. As explained above, ALK mutations are important targets in the treatment of neuroblastoma. A number of oncogene-specific small-molecule tyrosine kinase inhibitors have been developed, and a phase 1 trial of the ALK inhibitor crizotinib was conducted in the Children’s Oncology Group in children with refractory neuroblastoma or other cancers driven by ALK rearrangements and mutations. Although there were some tumors that respond to this drug, responses in children with ALK-mutated neuroblastoma were less frequent than patients with other types of cancers with ALK mutations. A combination of crizotinib with chemotherapy commonly used in treating newly diagnosed patients with high-risk neuroblastoma has been shown to enhance the sensitivity to ALK inhibition. A recent report of an ongoing NANT phase 1 trial testing the 3rd generation ALK inhibitor lorlatinib in patients with relapsed or refractory disease demonstrated a response rate of 30% for patients younger than 18 years old and 67% for patients 18 or older. The combination of chemotherapy and lorlatinib led to response rates of 63% among patients younger than 18 years old. Lorlatinib is also being tested in a phase 3 clinical trial in combination with standard of care treatment in patients with newly diagnosed neuroblastoma with ALK mutations. Anti-neuroblastoma activity has also been demonstrated in laboratory studies using an ALK antibody-drug conjugate.
-
Retinoids. Fenretinide (4-HPR) has shown effectiveness against neuroblastoma in a laboratory setting. Newer versions of this drug are in development to make it easier to give this medication to young children.
-
Angiogenesis inhibitors. Anti-angiogenesis therapy is focused on stopping angiogenesis, which is the process of making new blood vessels from existing blood vessels. Because a tumor needs the nutrients delivered by blood vessels to grow and spread, the goal of anti-angiogenesis therapies is to “starve” the tumor. Promising responses have been reported by the European (SIOPEN) BEACON clinical trial in patients with recurrent or refractory disease with temosolomide and topotecan combined with the anti-angiogenic drug bevacizumab.
-
TKIs. TKIs are a group of drugs that block cell communication and can stop tumor growth. Drugs that inhibit ALK are being tested in clinical trials. Other tyrosine kinase inhibitors that are being tested in clinical trials include inhibitors of the epidermal growth factor receptor (EGFR) and the RAS-MAPK signaling pathway. These receptors help tumor cells grow, and blocking them may slow or stop neuroblastoma growth.
-
Aurora kinase inhibitors. Aurora A kinase helps cells divide early on and is found in all cells that are dividing. Aurora kinase inhibitors are drugs that block this protein, stopping or slowing the cells from dividing. Inhibition of Aurora A kinase also destabilizes the MYCN protein. Early-phase trials testing an Aurora A kinase inhibitor in combination with irinotecan and temozolomide showed promising activity for recurrent neuroblastoma.
-
Combination chemotherapy. The drugs irinotecan (Camptosar) and topotecan are often used early when there is a recurrence. The combination of irinotecan and temozolomide (Methazolastone, Temodar) has few side effects. This chemotherapy treatment regimen has been evaluated in a Children’s Oncology Group study. Some recurrent tumors respond to this treatment. Other chemotherapy regimens have been used when there is a recurrence, including topotecan and low-dose cyclophosphamide.
-
ODC inhibitor. The ODC inhibitor eflornithine is FDA approved as a treatment after immunotherapy to reduce the risk of relapse in adults and children with newly diagnosed high-risk neuroblastoma. Eflornithine is also being tested in early-phase neuroblastoma clinical trials. The Children’s Oncology Group is testing the efficacy of the combination of eflornithine and chemotherapy in children with recurrent neuroblastoma.
-
Other treatment options. Demethylating drugs such as decitabine (Dacogen) and histone deacetylase inhibitors, which are substances that can prevent a tumor from growing and spreading, are also being tested for childhood neuroblastoma.
The NANT phase I consortium has a number of open clinical trials testing new treatments for children with recurrent or refractory neuroblastoma. (Please note this link takes you to a separate, independent website.) Several studies are testing combination therapy that includes immunotherapy plus chemotherapy. Always talk with your doctor about all of your child's treatment options, including clinical trials.
Whichever treatment plan you choose, palliative and supportive care will be important for relieving symptoms and side effects.
When a child has a recurrent tumor, family members often experience emotions such as disbelief or fear. Families are encouraged to talk with the health care team about these feelings and ask about support services to help them cope. Learn more about dealing with cancer recurrence.
Return to top
If treatment does not work
Although treatment is successful for many children with cancer, sometimes it is not. If a child’s neuroblastoma cannot be cured or controlled, this is called an advanced or terminal cancer. This diagnosis is stressful, and advanced cancer may be difficult to discuss. However, it is important to have open and honest conversations with your child’s health care team to express your family’s feelings, preferences, and concerns. The health care team has special skills, experience, and knowledge to support patients and their families and is there to help.
Hospice care is designed to provide the best possible quality of life for people who are expected to live less than 6 months. Parents and guardians are encouraged to talk with the health care team about hospice options, which include hospice care at home, a special hospice center, or other health care locations. Nursing care and special equipment can make staying at home a workable option for many families.
Some children may be happier and more comfortable if they can attend school part-time or keep up other activities and social connections. The child’s health care team can help parents or guardians decide on an appropriate level of activity. Making sure a child is physically comfortable and free from pain is extremely important as part of end-of-life care. Learn more about caring for a terminally ill child and advanced cancer care planning.
The death of a child is an enormous tragedy, and families may need support to help them cope with the loss. Pediatric cancer centers often have professional staff and support groups to help with the process of grieving. Learn more on grieving the loss of a child.
Return to top
The next section in this guide is About Clinical Trials. It offers more information about research studies that are focused on finding better ways to care for people with cancer. Use the menu to choose a different section to read in this guide.